Human Research Protection Program
 The Human Research Protection Program (HRPP) provides oversight for all research activities involving human participants at the University of Wisconsin–Madison. The HRPP is not an office, but rather a collective effort of all who participate in the conduct, review, approval, and facilitation of human participants research at UW–Madison.
The Human Research Protection Program (HRPP) provides oversight for all research activities involving human participants at the University of Wisconsin–Madison. The HRPP is not an office, but rather a collective effort of all who participate in the conduct, review, approval, and facilitation of human participants research at UW–Madison.
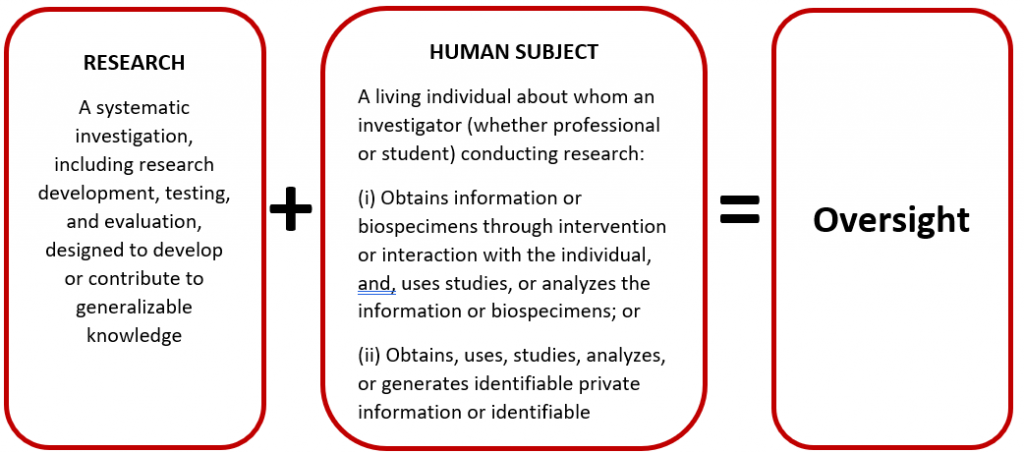
Is an activity Research involving Human Subjects?
- Refer to the Office of Human Research Protections (OHRP)'s Chart 1 to help determine whether an activity may require IRB review.
- Determining whether a project constitutes human subjects research rather than quality improvement or program evaluation involves multiple factors. If the project involves some characteristics of a research project, submission to the IRB for review is expected. To address the issue of documentation, the IRBs have developed decision tools that can provide self-certification that the project does not require IRB review and oversight.
- The HRPP Toolkit (including the Investigator Manual) describes when UW-Madison is considered to be engaged in human participants research and outlines certain activities that do not meet the definition of human participants research but nonetheless require UW–Madison IRB oversight.
Investigator Responsibilities
Investigator Manual
Reliance Manual
Data Storage & Retention
Policy on Data Stewardship, Access, and Retention
The University of Wisconsin–Madison is committed to protecting the rights and welfare of individuals participating as subjects in its research. The campus has two Institutional Review Boards (IRBs):
The Health Sciences IRB: Reviews more than minimal risk biomedical research, including FDA regulated research, VA research, and emergency use applications.
The Minimal Risk Research IRB: Reviews minimal risk research, including educational, social, behavioral, medical records review, and minimal biomedical interventional research.
Institutional Official
The Institutional Official (IO) is ultimately responsible for ensuring the protection of human participants at UW–Madison and maintaining oversight of the entire UW–Madison HRPP. There are two main components of the HRPP that provide administrative support and oversight of human participants research on campus: the IRBs/IRBs Office & the Office of Research Compliance.
Office of Research Compliance
The Office of Research Compliance (ORC) within the Office of the Vice Chancellor for Research & Graduate Education oversees a range of HRPP administrative and compliance activities including reliance and navigation, post-approval monitoring, ClinicalTrials.gov registration and reporting, AAHRPP accreditation activities, and quality assurance oversight. The ORC supports key advisory committees to the IO including the Cross Campus HRPP Committee and the Quality and Compliance Oversight and Advisory Committee (QCOAC). Working groups to support the HRPP advisory committees and HRPP program include the Quality and Compliance Operations Committee (QCOC), the HRPP Policy Revision working group, the HRPP Education working group, the ClinicalTrials.gov committee, and the Post-Approval Monitoring working group.
Institutional Review Boards (IRBs)
The IRBs oversee human subjects research conducted at UW-Madison. This includes research conducted off-site by University faculty and staff when acting as University employees or in connection with their University appointments. The IRBs review and oversee research to ensure that it meets ethical principles and complies with federal regulations, state laws, and university policies. The IRBs are composed of members from various disciplines in the medical, social, and behavioral sciences as well as community members. The IRBs are supported by the IRBs Office staff.
