HRPP Newsletter May 2022
Updated PI Status Policy
The Principal Investigator Status policy was recently updated. Major changes include:
- Visiting faculty may no longer serve as PI. Exceptions exist for visiting faculty who are in the process of obtaining a permanent appointment; exceptions are granted on a case-by-case basis by the institutional official.
- Research-professor track appointments may now serve as PI without additional approval from the Chair or Director.
ARROW Application Tip
As a reminder, ARROW applications include "More Information" icons that, when clicked, reveal helpful reminders and explanations for how to appropriately answer application questions. Clicking the "Always Show?" box will keep the "More Information" visible. 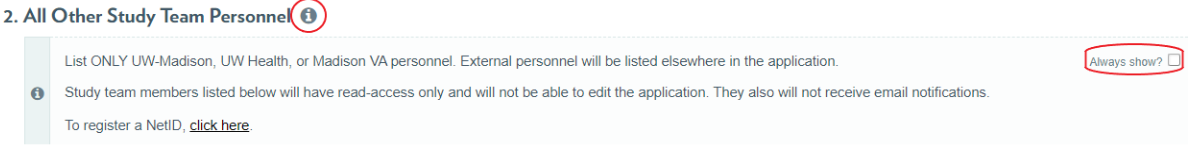
Looking for help answering the new ClinicalTrials.gov questions in ARROW?
Recent updates to the ClinicalTrials.gov section in ARROW may have left you with more questions than answers: Does my study really include an intervention? What’s a ‘health-related outcome’? Does all NIH funded research need to register with ClinicalTrials.gov? UW HRPP has resources to help you answer all of these questions, starting with the “More Information” icon mentioned above, right on the ClinicalTrials.gov page in the IRB applications. We also provide a KnowledgeBase page devoted to ClinicalTrials.gov registration, as well as a *brand new* website for the Clinical Research Office’s ClinicalTrials.gov service line that’s chock-full of useful information. If you still have questions (or just prefer to interface with a human), you can always contact ClincialTrials.gov compliance at either clinicaltrials.gov_support@research.wisc.edu or 608-890-1241.
Consent form statement on ClinicalTrials.gov changing for some studies
You’ve probably seen it a hundred times:
“A description of this clinical trial will be available on http://www.ClinicalTrials.gov, as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.”
This statement must appear somewhere in every consent form when your research is considered an Applicable Clinical Trial (ACT) by the FDA. But what if you are registering your study on ClinicalTrials.gov for some other reason? Starting this month, IRB reviewers and the ClinicalTrials.gov Review Committee will start requesting PIs use a slightly different statement for their non-ACT studies registering with ClinicalTrials.gov. This new statement will not reference U.S. law, as only ACTs are required by law to create and maintain a record on ClinicalTrials.gov (some studies must register as a condition of their grant, for publication purposes, etc.).
NEW: Single IRB Plan Template
Grant applications to NIH and other federal agencies ask for a single IRB plan when the grant involves multisite, non-exempt human subjects research. The reliance and navigation team (RELIANT) has developed a single IRB plan template that researchers can use when drafting their grant applications. Please email irbreliance@wisc.edu to request the template. RELIANT will be happy to assist you with any questions regarding single IRB review for your proposal.
AAHRPP Site Visit Updates
As mentioned in the January newsletter, the UW-Madison HRPP is currently undergoing re-accreditation through AAHRPP. The site visit portion of the re-accreditation process will occur in June and individuals from the UW-Madison human subjects research community will be interviewed. The individuals who have been selected by AAHRPP to be interviewed have been notified by the Office of Research Compliance and will receive materials to help them prepare for their interviews. More information about the re-accreditation will be made available in future HRPP newsletters.
Educational and Professional Development Resources
The Professional Research Education Program’s (PREP) webpage and the IRB’s webpage host video recordings of past educational offerings. These cover a variety of topics relevant to conducting human subjects research at UW-Madison and can be viewed at any time to assist with new training or as a refresher.
Registration is now open for "Recruiting via Social Media", which will be held virtually on 5/25 from 1:00-2:30 pm (CT). Click on the link to learn more and register.
