HRPP Newsletter – September 2025
New staff
We’re happy to announce that the IRB’s Minimal Risk Research team is welcoming a new analyst this month.
Jamie Hawkins has a bachelor’s in sociology, as well as a master’s in human ecology from UW-Madison. She previously worked as a consultant for Co-Create, doing collaborative research and evaluation projects and worked as a project assistant at the UW Child Development Lab and the Wisconsin HOPE Lab.
We’re excited to have Jamie bringing her skills and experience to the IRB Office. Welcome, Jamie!
CoCs for Non-NIH-Funded Research
The NIH Certificate of Confidentiality (CoC) online application system for non-NIH funded research has re-opened (see our past newsletter blurb about its temporary closure). Study teams collecting highly sensitive data can now request a CoC. If a CoC is issued, the study's ARROW application should be updated to note the receipt of the CoC and update consent language, as needed.
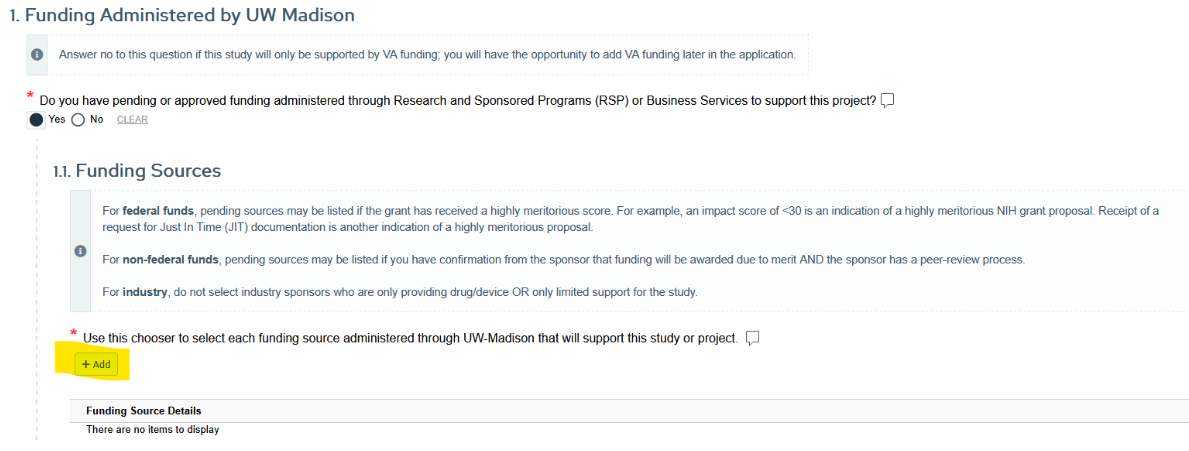
Linking Funding between ARROW and RAMP
All external funding supporting your study must be selected in ARROW by using the “Add a Funding Source” button in Section 1.1: Funding Sources. This includes all pending or approved federal, non-federal, and eligible industry funding administered through UW–Madison in RAMP. NOTE: funding from the Wisconsin Partnership Program (WPP) is also now in RAMP and selectable in section 1.1.
Quick guide:
- Create funding proposal in RAMP (you do not need to have IRB approval at this point) and submit to sponsor.
- When the proposal receives a fundable score, update or create an IRB application to include research described in funding and select the funding proposal in section 1.1 of the IRB application.
- During award set-up, update RAMP compliance questions 1.a to say YES you have submitted or have an approved IRB application. RAMP will automatically import the IRB data with current status of the protocol.
Please note: Only internal UW–Madison funding, excluding funding from WPP, should be listed in Question 2 of the funding section.
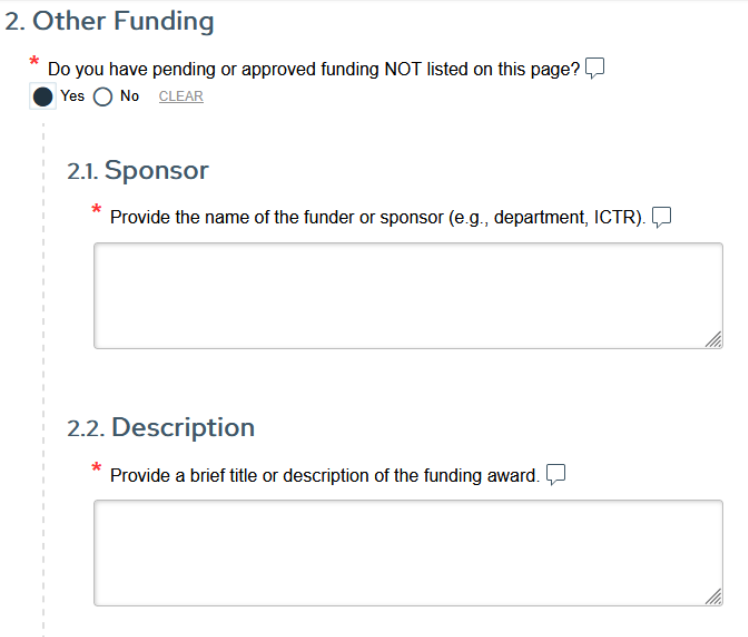
Accurate funding entry ensures compliance and smooth review!
Call for Non-Scientific IRB Members
Federal regulations require that every IRB includes at least one member whose primary concerns are in non-scientific areas. This person is known as the non-scientific member, and they play a critical role in protecting the rights and welfare of research participants.
To qualify as a non-scientific member, individuals typically do not hold a degree in a scientific field (such as biology, chemistry, or medicine) and do not have a professional background in scientific or biomedical work. Individuals with an undergraduate degree in a scientific field but who have never worked in a scientific or biomedical profession may qualify as non-scientific.
This role is ideal for people with experience in areas like law, social services, business, or community advocacy—anyone who can help ensure research is understandable and respectful to the public. If you or anyone you know is interested in serving in this role, please contact the IRB Office (IRBdirector@hsirb.wisc.edu). More information on serving as an IRB member can be found here.
ICTR's Workshop Series
Join ICTR’s Recruitment & Retention Resource Center this fall and winter for a virtual workshop series: “How We Talk With Research Participants: The Art of Communication”.
Each session of this five-part virtual series covers practical communication techniques that you can readily apply in your work to build stronger relationships with research participants and community members, increase understanding, and to improve recruitment and retention outcomes. Note: You do not need to attend all five sessions. Please select the sessions that are of interest to you.
