HRPP Newsletter April 2021
New Combined Website Now Live!
An important component of the IRB Efficiency Project (IEP) is the unification of the IRB offices under one administrative structure to align HRPP-related business processes and accountability. With this move, a new website to house information for all UW-Madison IRBs has been launched!
In addition to news items, upcoming education and outreach events, and submission resources, the new website will house the complete Toolkit Library. The Toolkit is a comprehensive set of documents for use by IRB staff, board members, and study teams. The Toolkit is comprised of worksheets, SOPs, and checklists that detail criteria for IRB approval, plus protocol and consent templates to help study teams create documents that meet those criteria.
While both the current Health Sciences IRBs and Educational and Social/Behavioral IRB KnowledgeBase sites will remain active for the next couple months, these will eventually be retired—along with most guidance documents— and the new website will be the sole source of content. The new Investigator Manual will replace much of the guidance from the previous sites: 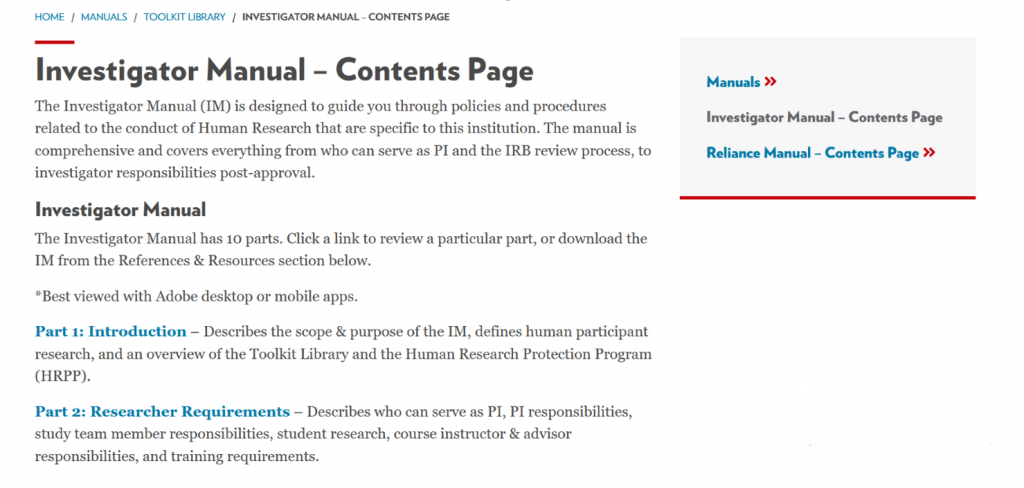
The Investigator Manual has 10 sections, all linked separately, or you can download the complete IM from the References & Resources section which is then searchable by keyword:

As you explore the new website, we encourage you to submit feedback via the Feedback Form accessible at the bottom of each page.
MRR and HS Board Restructuring and Leadership Changes
NEW Minimal Risk Research IRB committee (MRR IRB)
Minimal risk research is changing- many UW investigators engage in research that does not strictly fall into a minimal risk biomedical vs education/social/behavioral research bucket. Additionally, due to the changes in the federal regulations governing human subjects research in 2018, there has been a significant decrease in the number of studies reviewed by the full board of either of the UW minimal risk IRBs (the MR IRB and the ED/SBS IRB). Therefore, in June, the ED/SBS and the MR IRB will merge into a new Minimal Risk Research IRB (MRR IRB). This committee will review all minimal risk research, including educational studies (k-12, college and medical school), social and behavioral studies, and most minimal risk biomedical studies. Any study that requires FDA oversight or is done at the VA or with VA funding will be reviewed by the HSIRB, regardless of risk level. The membership of the committee will be drawn from the current ED/SBS and MR IRB committees. The committee will meet twice a month to ensure timely review of protocols.
Transition
During April and May, the MR IRB and ED/SBS IRBs will meet per their usual schedule. Beginning in June, all studies previously assigned to the ED/SBS IRB and most studies previously assigned to the MR IRB will be reviewed by the MRR IRB.
MRR IRB Leadership
- Mark Copelovitch, Professor in Political Science and Public Affairs and the current chair of the ED/SBS IRB will become chair of the new MRR until September 1, 2021, when he was scheduled to transition from this leadership position. Ed Hubbard, Associate Professor of Educational Psychology, the current ED/SBS Vice Chair will transition to the role of chair of the MRR.
- We are pleased to announce, that Kristina Matkowskyj, Associate Professor in the Department of Pathology and Laboratory Medicine, a current member of the MR IRB will be appointed as Vice Chair for the MR and then new MRR IRB beginning in April 2021.
HS IRB Updates
The HS IRB will continue to review all more than minimal risk biomedical research, including clinical trials, clinical research, and FDA-regulated research. All VA studies, regardless of risk will be reviewed by the HS IRB.
HS IRB Meeting capacity
Beginning in May, the Health Sciences (HS) IRB committee will increase the number of meetings from 4 to 6 meetings a month and create smaller, cohesive panels of members attending one of the 6-monthly meetings. Each of the 6 panels will be supported by an IRB Administrator and led by the HS IRB Chair or one of 4 new Vice Chairs.
HS IRB Leadership changes:
Starting in April, Michael Peterson, Associate Professor in the Department of Psychiatry, will be stepping down from his role as HS IRB Chair. Peter Rahko, Professor, Department of Medicine, Division of Cardiovascular Medicine, who has chaired the MR IRB for 17 years, will assume the role of HS IRB Chair.
Additionally, the HS IRB will have 4 new vice chairs:
- Donna Blankenbaker, Professor, Department of Radiology
- Sameer Mathur, Associate Professor, Department of Medicine, Allergy and Immunology
- Rowan Karaman, Assistant Professor, Department of Medicine, Endocrinology, CRU Medical Director and Research Subject Advocate
- Michelle Ciucci, Associate Professor, Communication Sciences & Disorders and Department of Surgery, Otolaryngology-Head and Neck Surgery
