HRPP Newsletter February 2023
Toolkit Revisions Live
The latest round of revisions went live at the beginning of this month. Updates to the Investigator Manual, Reliance Manual, and various templates were made. Below are some highlights of the updates:
- Protocol templates for Biomedical Studies (HRP-503) and Registries and Repositories (HRP-503a), as well as the consent template (HRP-502), have been updated to address information required as part of the IRB review of data shared under the NIH Data Management and Sharing policy, which went into effect January 25th.
- A new section addressing data and safety monitoring plans has been added to the Investigator Manual.
- A new section related to tribal research was added to the Investigator Manual. This section has helpful information and links to resources when conducting tribal research.
Additionally, updates were made to Toolkit checklists and worksheets that IRB Staff have been using to conduct reviews. As a reminder, we welcome feedback via the Toolkit Feedback Form that is linked throughout the IRB website or by email (toolkit@research.wisc.edu).
RETIRING IRB KNOWLEDGEBASE (KB) SITES
We are nearly two years into the launch of the IRBs Office website which includes the Toolkit library, consisting of a comprehensive investigator manual, and updated guidance. Much of the information from the KB sites has been incorporated into the investigator manual, toolkit documents or guidance on our website. Therefore, the KB sites will no longer be available as of March 1, 2023.
If you have any feedback on our current website, we welcome your comments. And, as noted above, any feedback regarding Toolkit documents is also welcome.
VA Status page updates in ARROW
The VA RDC (Research and Development Committee) may grant permission for some non-VA studies to conduct limited activities involving the VA, such as recruitment of veterans to a study not focused on veterans’ health, or use of VA space for administrative or sample process activities only, without these studies falling under the purview of the VA RDC.
New questions on the VA page of the ARROW application will allow study teams to choose this option if it applies to their studies. Activities selected here must first be approved by the VA R&D committee, but studies limited to these activities at or with the VA are NOT considered VA human subjects research and will not be subject to continued VA oversight beyond the initial approval.
New Community-Engaged Research CITI modules Available
Two new elective modules have been added to the UW Human Subjects Protections (HSP) Course: “Introduction to Community-Engaged Research (CEnR)” and “Introduction to Community-Based Participatory Research (CBPR).” These modules introduce approaches to incorporating input from community partners throughout the research process. “Introduction To Community-Engaged Research (CEnR)” is recommended for individuals new to or interested in learning more about engaging community partners in research, while “Introduction to Community-Based Participatory Research (CBPR)” is recommended for individuals interested in incorporating CBPR strategies throughout the research process.
Learners can also complete the Community Research Course, which is a supplemental training that can be added by learners at any time by logging in through the UW CITI Portal, selecting “View Courses” for University of Wisconsin – Madison, clicking “Add a Course”, and selecting “Community Research” under Question 4.
Upcoming Education Opportunities
PREP: Documenting Consent: When, Why, and How, 3/9 from 11am-12pm
Presenters will go over key considerations for documenting informed consent: what “counts” as documentation, which regulatory determinations are required and how to request them, remote consent processes, exemption requirements, and what to consider if assent, HIPAA, or FDA regulations apply. There will also be time for Q&A.
PREP: Self-Audit Workshop (for non-FDA-regulated studies), 3/14 from 10-11:30am
Protocol adherence and excellent recording keeping aren’t just about compliance. They help ensure the protection of participants’ safety and confidentiality and accurate data capture for scientific publications. Self-audits are a practical and accessible way to check in on study conduct, identify issues, and improve processes. This presentation will introduce study teams to self-auditing. The presenters will identify things to consider when developing a self-audit plan, explain how to conduct a self-audit, and describe common audit findings. Attendees will then practice self-auditing during a hands-on mock audit.
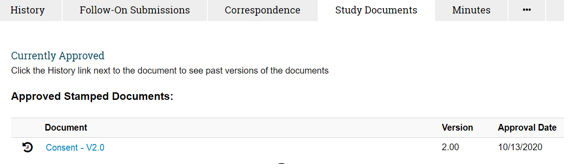
Reminder to Use Stamped Consent Documents
For studies approved by a UW-Madison IRB, the stamped consent document must be used when obtaining written consent on a physical form. Once a consent form is approved by the IRB, it is stamped on the bottom right of each page with an approval date and can be obtained from the “Study Documents” tab in ARROW (see below). Obtaining stamped consent forms from the “Study Documents” tab also helps ensure you are using the most recently approved version.